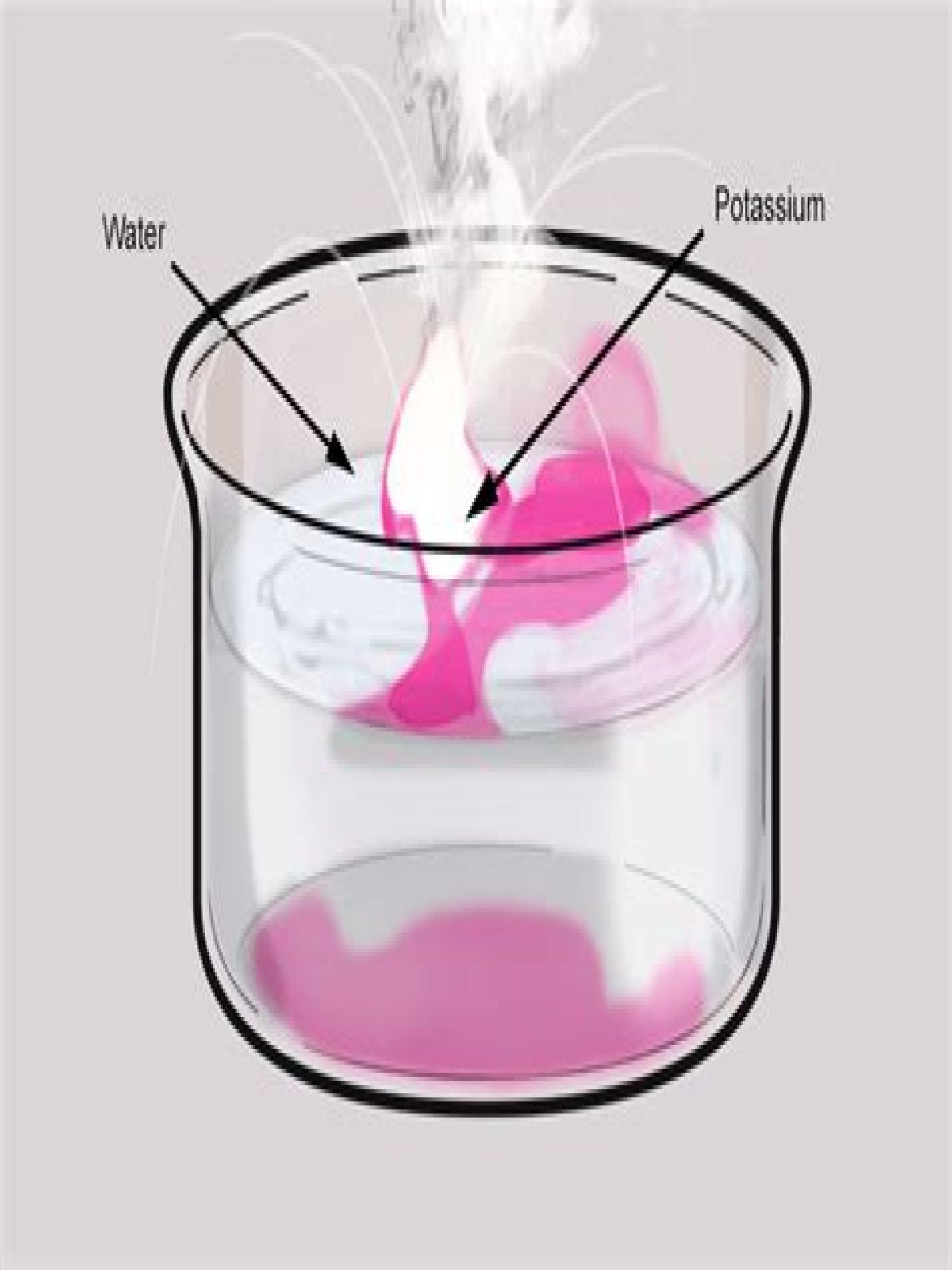
The molten metal spreads over the water and exposes a larger surface to water. Also, the hydrated radius of lithium is the greatest out of all alkali metals. This reduces the ionic mobility which in turn reduces the speed of the molten metal. That’s why potassium gives a more violent reaction with water.
- Why does potassium react with water?
- Does potassium react with water violently?
- Why does potassium react more violently with water than sodium?
- Why does potassium react more violently than calcium?
- Why does potassium react vigorously with cold water?
- What reacts violently with water?
- Why is potassium more reactive in water than lithium?
- Why is potassium more reactive?
- How the reaction of potassium with water compares with the reaction of lithium with water?
- Why is potassium K more energetic in its reaction with water than sodium Na or lithium Li )?
- Is potassium chloride water reactive?
- Which reacts faster with water lithium or potassium?
- What is the chemical equation for potassium and water?
- Does potassium react with hot water?
- What reacts most vigorously with cold water?
- Why is the reaction of calcium with water less violent?
- How does the reactivity of potassium with water differ from that of sodium with water?
- Why is potassium more reactive than sodium GCSE?
- Why potassium is more reactive than sodium?
- Why is potassium more reactive than lithium GCSE?
- Does potassium fizz in water?
- Why does cesium explode in water?
- Why does lithium explode in water?
- What will happen if sodium and potassium reacts with water?
- Do sodium and potassium react with water?
- Does potassium follow water?
- When potassium reacts with water then the gas evolved is?
Why does potassium react with water?
Potassium compounds, when dissolved in water, form Potassium ions, positively changed Potassium atoms, vital to our bodies functions. Potassium metal reacts violently with water (A compound of Hydrogen and oxygen) to release Hydrogen gas, leaving another compound behind, Potassium Hydroxide.
Does potassium react with water violently?
Potassium reacts violently with water to produce half a mole of hydrogen per mole of potassium and water and generates approximately 47 kilocalories per mole of heat. Potassium can be stored in nitrogen gas with no reaction. It reacts with hydrogen at approximately 350 °C (660 °F) to form the hydride.
Why does potassium react more violently with water than sodium?
As potassium is larger than sodium, potassium’s valence electron is at a greater distance from the attractive nucleus and is so removed more easily than sodium’s valence electron. As it is removed more easily, it requires less energy, and can be said to be more reactive.Why does potassium react more violently than calcium?
Metallic ch. increases from left to right so potassium has got greater metallic ch. than alcium and since metals have reducing ch. … Alkali metals have got the highest reactivity and hence potassium being one has higer reactivity than calcium which is an alkaline earth metal.
Why does potassium react vigorously with cold water?
Because pottasium is a highly reactive metal So it reacts vigorously with even cold water .
What reacts violently with water?
Chemical NameReaction with WaterPotassium amideViolent reaction which may cause ignitionPotassium hydrideReleases hydrogen gasPotassium metalForms KOH and hydrogen gasSilicon tetrachlorideViolent reaction producing silicic acid
Why is potassium more reactive in water than lithium?
Potassium metal is indeed more reactive than lithium metal, because potassium has a more loosely bound valence electron. In direct reactions, potassium reacts more violently than lithium.Why is potassium more reactive?
All the group 1 metals are reactive, but they get more reactive as you go down the group, so potassium is more reactive than sodium, which is more reactive than lithium. This can be explained by looking at the electronic structure of the atoms: In order to react, the metal needs to lose an electron.
Why does potassium melt into a ball in water?enthalpy change (kJ / mol)Li-222Na-184K-196Rb-195
Article first time published onHow the reaction of potassium with water compares with the reaction of lithium with water?
In this dramatic demonstration, lithium, sodium, and potassium react with water to produce hydrogen gas and the hydroxides of the metals. Lithium reacts fairly slowly, fizzing. … The potassium reacts violently, immediately bursting into a flame which has the characteristic violet color of potassium.
Why is potassium K more energetic in its reaction with water than sodium Na or lithium Li )?
Li kinda sizzles in water, Sodium really reacts quickly, and Potassium reacts so rapidly and produces so much more heat, the released Hydrogen actually ignites.
Is potassium chloride water reactive?
Hygroscopic. Water soluble. POTASSIUM CHLORIDE is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture (NTP, 1992).
Which reacts faster with water lithium or potassium?
The reactivity of the active metals can be demonstrated by dropping pieces of lithium, sodium, and potassium into water. Lithium reacts slowly with water, sodium reacts much more rapidly, and potassium reacts violently.
What is the chemical equation for potassium and water?
By applying hit and trial method the balanced equation of the given reaction is: 2K(s)+2H2O(l)→2KOH (aq)+H2(g) If 3.0 moles of potassium react with excess water, what volume of hydrogen gas will be produced?
Does potassium react with hot water?
When potassium is added to water, the metal melts and floats. It moves around very quickly on the surface of the water. The hydrogen ignites instantly. The metal is also set on fire, with sparks and a lilac flame.
What reacts most vigorously with cold water?
The alkali metals (Li, Na, K, Rb, Cs, and Fr) are the most reactive metals in the periodic table – they all react vigorously or even explosively with cold water, resulting in the displacement of hydrogen.
Why is the reaction of calcium with water less violent?
Calcium reacts with water to form calcium hydroxide and hydrogen. The heat produced in this reaction is less which is insufficient to burn the hydrogen gas which is formed. Hence, the reaction of calcium with water is less violent.
How does the reactivity of potassium with water differ from that of sodium with water?
The reaction of potassium and water is more vigorous than sodium’s: fizzing (hydrogen gas is released) potassium floats and moves around on the water. catches fire with a LILAC flame.
Why is potassium more reactive than sodium GCSE?
Therefore, potassium has an additional shell of electrons and thus 8 more electrons. This extra shell of electrons shields the attractive force exerted on the outer electron by the nucleus. As a result, less energy is required to remove the outer electron of potassium and so it is more reactive.
Why potassium is more reactive than sodium?
But on the other hand, potassium atoms due to being larger in size than the sodium atom has low ionization energy and thus, they can lose electrons easily and are less stable and more reactive.
Why is potassium more reactive than lithium GCSE?
Potassium is more reactive than lithium although they both need to lose only one electron to have full outer shells. This is because the outer electron of potassium atom is further from the positive attractions of the nucleus compared to the outer electron of lithium.
Does potassium fizz in water?
This is because metal hydroxides are alkaline. Lithium: Fizzes around the surface of the water. Sodium: Melts to form a small ball, and then fizzes rapidly. Potassium: Quickly melts to form a ball, burns violently with sparks and a lilac flame, disappearing rapidly, often with a small explosion.
Why does cesium explode in water?
Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. The reaction is so explosive that it often shatters the container. The sublimation energy (1) is the smallest of the alkali metals because the Cs atoms are the biggest.
Why does lithium explode in water?
Lithium reacts intensely with water, forming lithium hydroxide and highly flammable hydrogen. The colourless solution is highly alkalic. … Depending on the application Molecular Sieves Desiccant is effective in preventing the presence of free water and hydrogen to lower explosive limits.
What will happen if sodium and potassium reacts with water?
As the piece of metal skitters across the surface of the water in a beaker and — particularly in the case of potassium — it appears to catch fire, it is not obvious that the explanation for both phenomena lies in the production of hydrogen gas.
Do sodium and potassium react with water?
For decades, science enthusiasts have delighted at the famously energetic way sodium and potassium explode on contact with water. … They recognized that the steam and hydrogen generated early on in the reaction should form a buffer layer over the metal surface and impede water from continuing to react.
Does potassium follow water?
Potassium is both an electrolyte and a mineral. It helps keep the water (the amount of fluid inside and outside the body’s cells) and electrolyte balance of the body. Potassium is also important in how nerves and muscles work. Potassium levels often change with sodium levels.
When potassium reacts with water then the gas evolved is?
ABSWER: Hitrogen gas is evolved when potassium reacts with water.