6.7 Factors Affecting SN1 Reactions. Just as with SN2 reactions, the nucleophile, solvent and leaving group also affect SN1 (Unimolecular Nucleophilic Substitution) reactions. Polar protic solvents have a hydrogen atom attached to an electronegative atom so the hydrogen is highly polarized.
- What makes a SN1 reaction go faster?
- What do SN1 reactions favor?
- Which halide reacts fastest in an SN1 reaction?
- What are the factors that influence SN1 and SN2 reactions?
- Which step in SN1 reaction is slow rate-determining step?
- Which compound will react fastest by SN1 mechanism?
- On what factors Nucleophilicity depends?
- Which compound reacts most rapidly by an SN1 mechanism?
- Which compound in the following pairs will react faster in SN1 reaction with OH & Why CH3CH2Cl or C6H5CH2Cl?
- Which of the following is not true for SN1 reaction?
- Which of the compounds will react faster in SN1 reaction with the OH ion ch3 ch2 Cl or c6h5 ch2 Cl and why?
- Which compound undergoes an SN1 substitution reaction?
- Which compound undergoes hydrolysis by SN1 mechanism at the fastest rate?
- What is mechanism of SN1 reaction?
- How many factors are of substantial importance for SN1 reaction?
- How does temperature affect SN1 reactions?
- How would doubling the concentration of the nucleophile affect the rate of an SN1 reaction?
- What conditions favor SN1 and E1 reactions?
- Which compound in each of the following pairs will react faster in an SN1 reaction with OH?
- Which compound out of the following pairs will react faster in SN1 reaction and why ch3ch2br or c6h5ch2br?
- Which have the least order of reactivity towards the SN1 reaction?
- Which statement is correct for an SN1 reaction at chiral carbon?
- Is rearrangement possible in SN1 reaction?
What makes a SN1 reaction go faster?
An SN1 reaction speeds up with a good leaving group. This is because the leaving group is involved in the rate-determining step. A good leaving group wants to leave so it breaks the C-Leaving Group bond faster.
What do SN1 reactions favor?
SN1 reactions are favored by polar protic solvents (H2O, ROH etc), and usually are solvolysis reactions. SN2 reactions are favored by polar aprotic solvents (acetone, DMSO, DMF etc).
Which halide reacts fastest in an SN1 reaction?
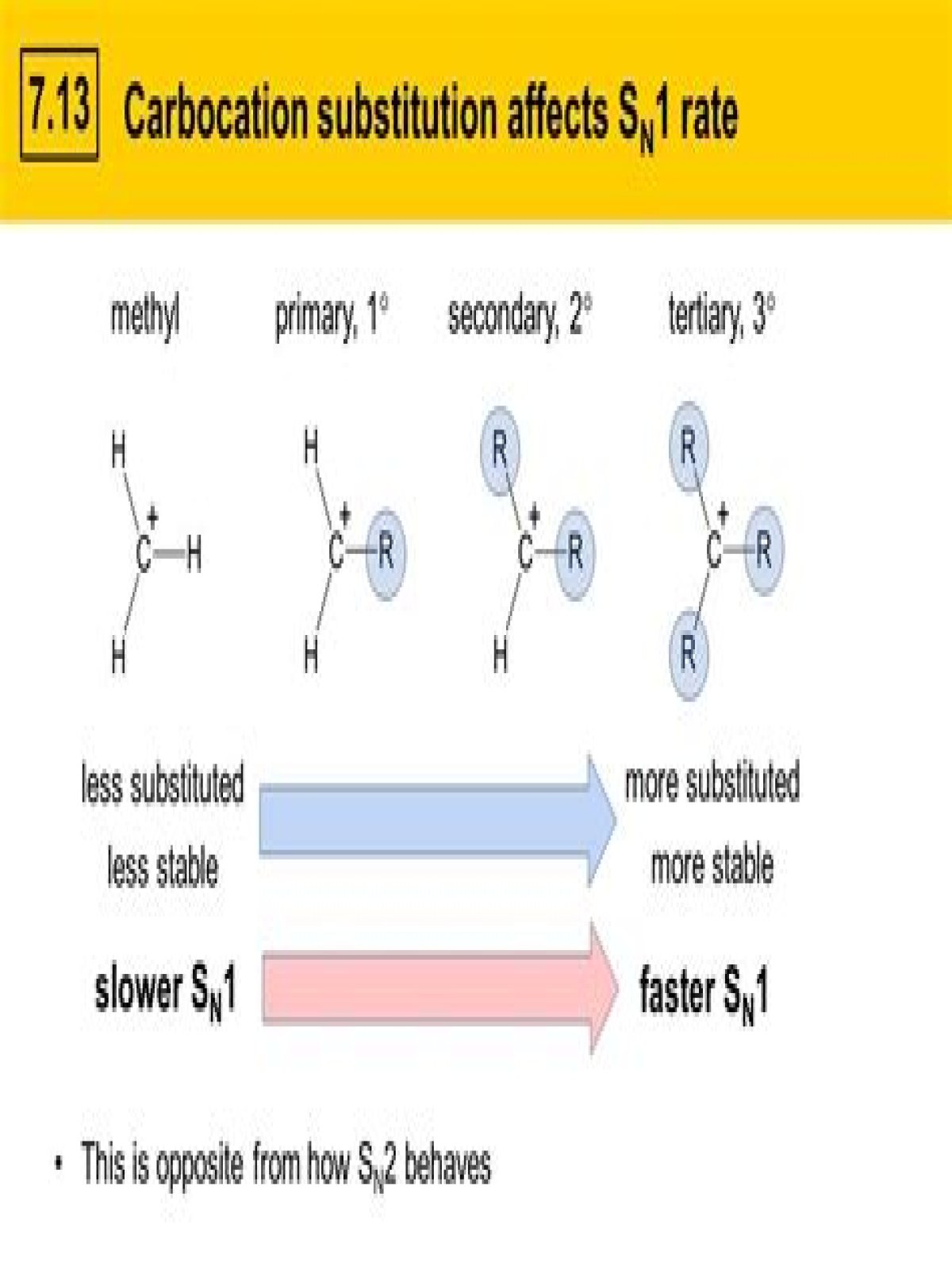
3. The Reaction Rate Increases With Substitution At Carbon. When we subtly change the types of substrates (e.g. alkyl halides) we use in these reactions, we find that tertiary substrates (for instance, t-butyl bromide) are considerably faster than secondary alkyl bromides, which are in turn faster than primary*.What are the factors that influence SN1 and SN2 reactions?
- Nature of substrate.
- Nucleophilicity of the reagent.
- Solvent polarity.
Which step in SN1 reaction is slow rate-determining step?
The formation of a carbocation is the slow, or rate-determining, step. The subsequent step, formation of a bond between the nucleophile and the carbocation, occurs very rapidly.
Which compound will react fastest by SN1 mechanism?
Detailed Solution. The correct answer is MeO – CH2 – Cl. MeO-CH2- Cl will react faster in an SN1 reaction with the OH- ion. This happens due to the stability of the carbocation in the compound.
On what factors Nucleophilicity depends?
Nucleophilicity depends on many factors, including charge, basicity, solvent, polarizability, and the nature of the substituents.Which compound reacts most rapidly by an SN1 mechanism?
(C6H5)3CCl is hydrolysed most rapidly by SN1 because (C6H5)3C+ is most stable.
Why is SN1 favored over E1?SN1 and E1 are grouped together because they always occur together. If the leaving group dissociates first, there is an equally likely chance of the nucleophile attacking (SN1) as there is the base pulling off the b-hydrogen (E1).
Article first time published onWhich compound in the following pairs will react faster in SN1 reaction with OH & Why CH3CH2Cl or C6H5CH2Cl?
The rate of SN1 reaction depends upon the stability of carbocation intermediate formed during the reaction. Hence C6H5CH2Cl will react faster due to the resonance stabilisation of carbocation. While in CH3CH2Cl carbocation is stabilised only through hyperconjugation.
Which of the following is not true for SN1 reaction?
In SN1 reactions, rate of the reaction depends upon the concentration of the substrate only. It does not depend upon the concentration of the nucleophile.
Which of the compounds will react faster in SN1 reaction with the OH ion ch3 ch2 Cl or c6h5 ch2 Cl and why?
Answer : C6H5— CH2— Cl will react faster in an SN1 reaction with the OH- ion. … C6H5 group is already stable due to resonance, and the CH2 attached will gain that stability, thus forming a stable C6H5CH2+ carbocation after the cleavage in the first step of the SN1 reaction.
Which compound undergoes an SN1 substitution reaction?
An example involves the reaction of 2-bromo methylpropane with NaOH in water. You get as a result methyl proapan-2-ol and NaBr. In SN1 reactions, the rate of the reaction will only depend on the concentration of the halogenoalkane.
Which compound undergoes hydrolysis by SN1 mechanism at the fastest rate?
Explanation: C6H5CH2Cl will undergo faster hydrolysis reaction by SN1 mechanism. This is because the carbocation formed from C6H5CH2Cl is resonance stabilized. SN1 mechanism is favored by a stable carbocation.
What is mechanism of SN1 reaction?
SN1 reaction mechanism follows a step-by-step process wherein first, the carbocation is formed from the removal of the leaving group. Then the carbocation is attacked by the nucleophile. Finally, the deprotonation of the protonated nucleophile takes place to give the required product.
How many factors are of substantial importance for SN1 reaction?
If you think about it, in a substitution reaction there really are two main factors that tell you whether it’s SN2 or SN1 : the leaving group propensity or the strength of an incoming nucleophile. Two molecules react, and one displaces a substituent on the other.
How does temperature affect SN1 reactions?
The higher the temperature, the faster a non-biological reaction tends to occur. For SN1 and SN2 reactions, the higher the temperature, the more elimination products you get. The more elimination products you get, since the amount of reactant is limited, the less substitution products you get, as well.
How would doubling the concentration of the nucleophile affect the rate of an SN1 reaction?
The rate equation of an SN1 reaction is: k [R-X]. Notice that the nucleophile is not in the rate equation. Therefore, doubling the concentration of the nucleophile will have no effect on the rate of the reaction. … Therefore, doubling the concentration of the nucleophile will double the rate of the reaction.
What conditions favor SN1 and E1 reactions?
In general, in order for an SN1 or E1 reaction to occur, the relevant carbocation intermediate must be relatively stable. Strong nucleophiles favor substitution, and strong bases, especially strong hindered bases (such as tert-butoxide) favor elimination.
Which compound in each of the following pairs will react faster in an SN1 reaction with OH?
Therefore, CH3I will react faster than CH3Br in SN2 reactions with OH-. The SN2 mechanism involves the attack of the nucleophile at the atom bearing the leaving group.
Which compound out of the following pairs will react faster in SN1 reaction and why ch3ch2br or c6h5ch2br?
SN1 mechanism involves formation of a carbocation. The more stable carbocation will be formed, more will the reaction be faster. So (CH3)3CBr would undergo faster SN1 type reaction.
Which have the least order of reactivity towards the SN1 reaction?
Explanation: When it comes to one reaction, CH X 3 − Br is the least reactive. In the case of CH X 3 − Br the carbocation generated, which is the least stable (1° carbocation), the reaction proceeds through the creation of carbocation.
Which statement is correct for an SN1 reaction at chiral carbon?
In an S_(N^(1)) reaction on chiral centres, there is. 100% racemisation. it is the correct answer.
Is rearrangement possible in SN1 reaction?
Recall that the first step in the SN1 is that the leaving group leaves to give a carbocation. … Therefore, a rearrangement can occur to give the more stable tertiary carbocation, which is then attacked by the nucleophile (water in this case).